Molar Mass Calculator
Calculate Molar Mass from Chemical Formula
Calculation Results
Interactive Periodic Table (for Reference - click an element)
What is Molar Mass & How to Calculate It
- Write the chemical formula distinctly, using element symbols and correct subscripts (e.g., C6H12O6).
- Find the atomic mass of each element from the periodic table (most abundant isotope by default).
- Multiply the atomic mass by the number of atoms for each element in the formula.
- Sum the total mass for all elements to get the compound’s molar mass.
Molar mass of H2O = (2 × 1.008) + (1 × 15.999) = 18.015 g/mol
Isotopes & Atomic Weights
- For most chemistry calculations, average atomic weights are sufficient and standard.
- For advanced or high-accuracy scenarios (especially analytical chemistry or isotope labeling), specific isotopic mass should be considered. Click an element in the periodic table for details.
Why Calculate Molar Mass?
- Stoichiometry: Convert between moles and grams in chemical equations.
- Solution preparation: Weigh accurate amounts of reagents for standards and reactions.
- Gas calculations: Use in PV=nRT for determining moles from mass and vice versa.
- Analytical chemistry: Calculate concentrations for titrations or spectroscopy.
- Isotope labeling: Track atomic substitutions in research or medical applications.
⚗️ Molar Mass Calculator: Accurately Calculate the Molecular Weight of Any Compound
Ever wondered how much a mole of a substance weighs? Whether you’re a student tackling chemistry homework or a scientist preparing a precise solution, the Molar Mass Calculator is your go-to tool for accurately determining the molecular weight of chemical compounds.
Understanding molar mass is essential in stoichiometry, solution preparation, and even pharmaceutical dosage calculation. With this calculator, complex chemistry becomes easy and accurate.
🔍 What Is Molar Mass?
Molar mass (also known as molecular weight) is the mass of one mole of a substance, expressed in grams per mole (g/mol). It’s calculated by adding together the atomic masses of each element in a compound, multiplied by the number of atoms of that element.
Example:
For water (H₂O),
- Hydrogen (H) = 1.008 g/mol × 2 = 2.016 g/mol
- Oxygen (O) = 16.00 g/mol × 1 = 16.00 g/mol
- Total Molar Mass = 18.016 g/mol
🧮 How Does the Molar Mass Calculator Work?
This tool automates the entire process:
- You input the chemical formula (e.g., NaCl, C6H12O6).
- It identifies each element and its subscript.
- The calculator fetches atomic weights from the periodic table.
- It sums up the contributions to get the total molar mass.
📊 Image Preview
Here’s a graphical view showing the mass contribution of each element in a sample molecule (C₆H₁₂O₆N):
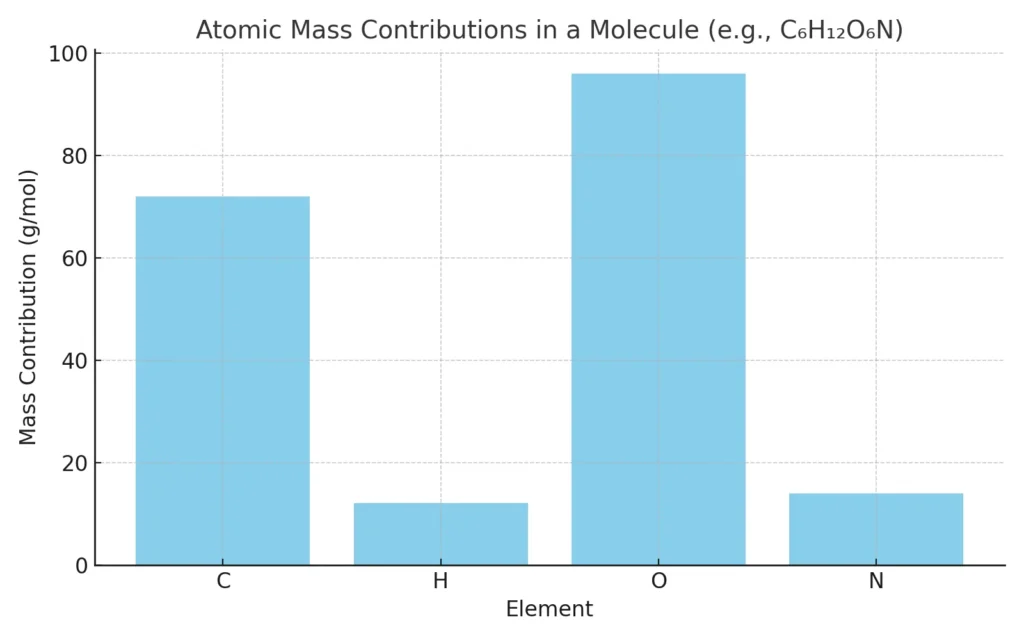
🧪 Use Cases for Molar Mass
| Field | Application |
|---|---|
| Chemistry | Stoichiometric calculations |
| Biology | Understanding biochemical reactions |
| Pharmacy | Drug dosage & formulation |
| Education | Lab experiments & reports |
| Manufacturing | Material formulation & chemical engineering |
📘 Example Calculations
1. Glucose (C₆H₁₂O₆)
- C: 12.01 × 6 = 72.06
- H: 1.008 × 12 = 12.096
- O: 16.00 × 6 = 96.00
- Total: 180.156 g/mol
2. Ammonia (NH₃)
- N: 14.01 × 1 = 14.01
- H: 1.008 × 3 = 3.024
- Total: 17.034 g/mol
🧠 Why Is Molar Mass Important?
Understanding molar mass allows you to:
- Convert grams ↔ moles for stoichiometry.
- Prepare accurate chemical solutions (molarity).
- Ensure proper dosing in pharmaceuticals.
- Perform limiting reagent calculations in reactions.
📈 Real-Time Applications
- 💊 Medicine: Dosage formulation based on molar concentrations.
- 🧬 Biochemistry: Quantifying DNA or protein samples.
- 🧫 Lab Experiments: Calculating reagent quantities.
- ⚙️ Industrial Chemistry: Bulk formulation in material processing.
🌐 External Resources
These sites offer interactive practice tools and deeper learning for students and professionals alike.
🔑 Features Included
- Molar Mass Calculator
- Calculate Molecular Weight
- Molar Mass of Compounds
- Atomic Mass Contribution
- Chemistry Molar Mass Tool
- How to Find Molecular Weight
- Stoichiometry Calculator
- Mass of 1 Mole of a Compound
✅ Summary Table
| Feature | Description |
|---|---|
| Input | Chemical formula (e.g., C₂H₆O) |
| Output | Molar mass in g/mol |
| Elements Supported | Full periodic table |
| Output Format | Instant result with breakdown |
| Applications | Chemistry, biology, pharmaceuticals, research |
🧪 Final Thoughts
The Molar Mass Calculator is more than just a tool — it’s a must-have companion for anyone working with chemicals. From classrooms to high-tech labs, it provides accuracy, speed, and ease-of-use in calculating molecular weights.

1 thought on “Molar Mass Calculator”